Our laboratory is engaged in various research projects, focusing on redox reactions of metal complexes and porphyrins inspired by biological phenomena. In particular, we are conducting research on reactions related to proton-coupled electron transfer (PCET), in which protons and electrons are transferred between molecules in a concerted or sequential manner. T research themes of our laboratory are broadly divided into three categories.
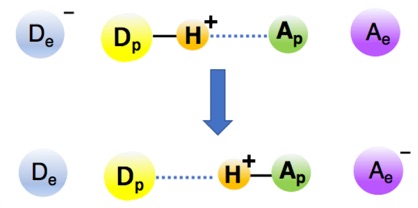
Schematic diagram of proton-coupled electron transfer:
De electron donor; Dp proton donor; Ae electron acceptor; Ap proton acceptor
The following paper summarizes PCET in metal complexes covered in these topics.
★T. Kojima, Bull. Chem. Soc. Jpn. 2020, 93, 1571-1582 (Award account).
See also: T. Kojima, J. Inorg. Biochem. 2025, 267, 112856.