Synthesis of inorganic nanoparticles in the nanospace of organic cage molecule
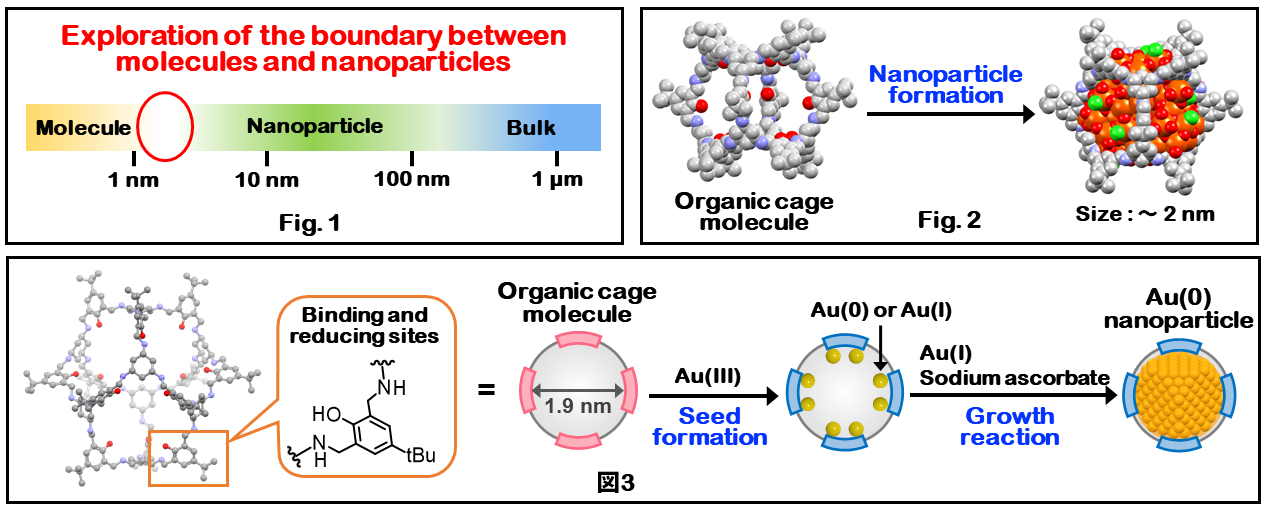
Inorganic nanoparticles such as metals and metal oxides are classified into bulk solids, nanoparticles, and clusters according to their size range, and are known to exhibit significantly different physical properties depending on their size (Fig. 1). In particular, nanoparticles in the size range of 1 to several nm, which is the boundary region between molecules and nanoparticles, have been found to exhibit unique properties associated with the high ratio of surface atoms and the emergence of quantum nature. Generally, metal oxides and metal nanoparticles are obtained by synthesizing nanoparticles in the presence of surface protectants to prevent overgrowth. However, nanoparticles in the size region of 1 - several nm easily aggregate, making it difficult to predict the size of the nanoparticles before synthesis. In particular, the general method to synthesize metal oxide nanoparticles with a size range of 1 - 2 nm has not been established.
We utilize self-assembled organic cage molecules as the platform for synthesizing inorganic nanoparticles. These cage molecules have the advantage that the size of the internal nanospace can be clearly defined by changing the structure of the component organic molecules, and that coordination sites for metal ions and functional units can be introduced by chemical modification of the cage molecule backbone. We have successfully synthesized iron oxide (ferrihydrite) nanoparticles with a particle size of about 2 nm by using organic cage molecules with chelate-type coordination sites in the inner space (Fig. 2, J. Am. Chem. Soc. 2018.). We also reported the synthesis of gold nanoparticles with a particle size of about 2 nm via seed formation and particle growth reactions in the inner space by using organic cage molecules with binding and reducing sites of metal ions (Fig. 3, Chem. Eur. J. 2023.).
Molecular Prussian blue analogues ―Unique properties of cyanide-bridged multinuclear complexes―
Prussian blue has an infinite structure in which FeII and FeIII ions are cross-linked by cyanide ions. They have been used as a pigment since ancient times because of their characteristic deep blue color derived from the electron transfer from FeII to FeIII ions. Recently, they have been studied as a material that exhibits electrochromism and a rechargeable battery.
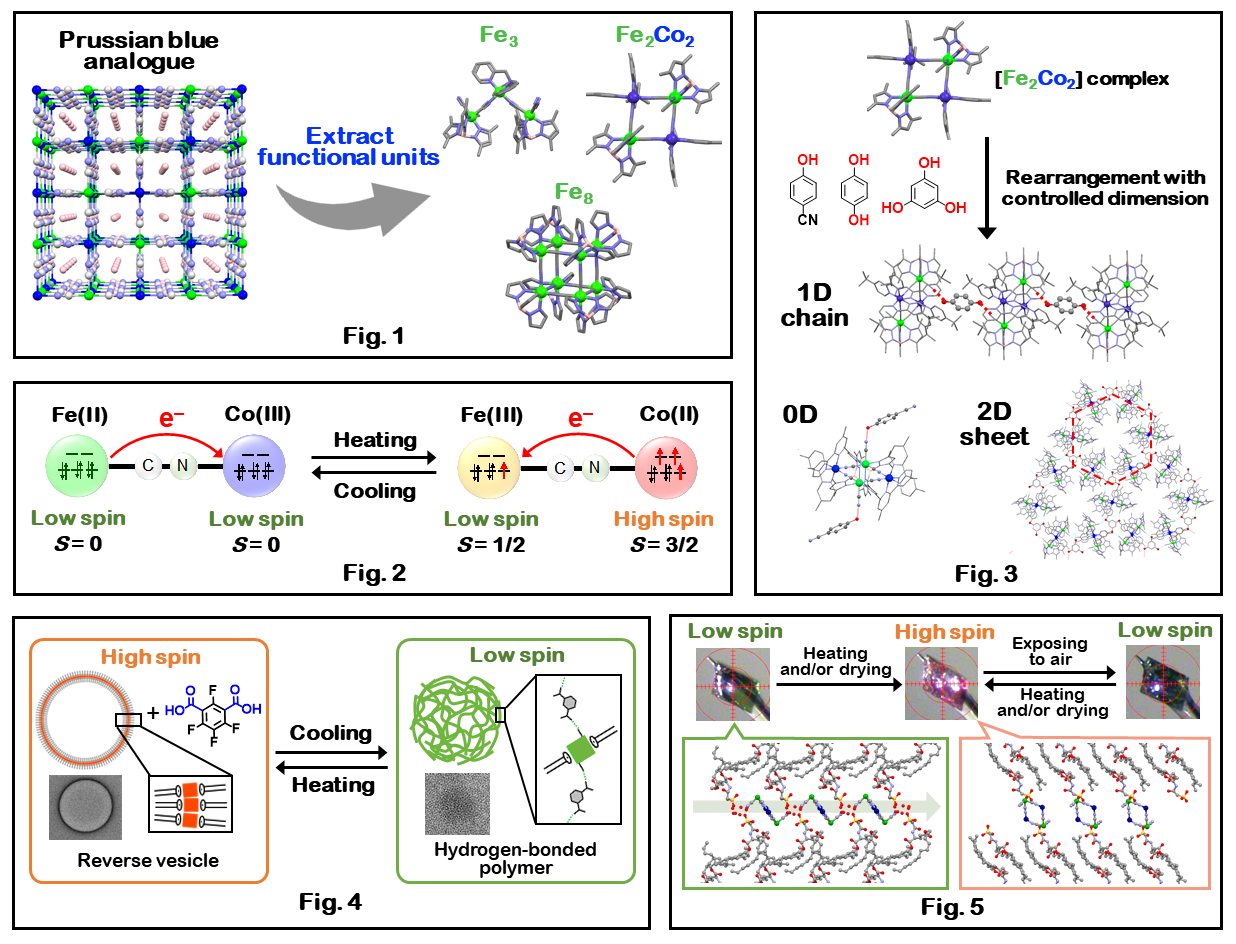
Our research focuses on cyanide-bridged multinuclear complexes, which can be regarded as functional molecular units extracted from analogues of Prussian blue (Fig. 1). Especially, electron-transfer-coupled spin transition (ETCST) occurs in cyanide-bridged Fe-Co complexes by applying heat and light. In the high-temperature phase, the complex is paramagnetic (FeIIILS-CoIIHS) with S = 1/2 + 3/2, but when the sample is cooled, electron transfer from cobalt ions to iron ions occurs and the complex turns diamagnetic (FeIILS-CoIIILS) (Fig. 2).
We have discovered the ETCST phenomenon in [Fe2Co2] square tetranuclear complexes and reported that a two-step phase transition occurs upon heating and cooling of the molecule (J. Am. Chem. Soc. 2011.). We have synthesized a variety of cyanide-bridged polynuclear complexes that have interesting properties. We have also reported rearrangements with controlled dimensionality of [Fe2Co2] complex units (Fig. 3). Network structures such as linear and sheet structures have been constructed by utilizing the hydrogen bonds between terminal cyanide groups of [Fe2Co2] complex and phenols (Angew. Chem. Int. Ed. 2017, Chem. Eur. J. 2017.).
We have also constructed two assemblies between the [Fe2Co2] complex and amphiphilic anions (Fig. 4 and Fig. 5). The first assembly exhibited an ETCST-triggered reversible structural change between reverse vesicle and hydrogen-bonded polymer in solution (Fig. 4, Chem. Eur. J. 2023.). The second assembly is crystalline with an alternating layered structure and exhibited a reversible structural change between the hydrogen-bonded 1D chain LS complex and the discrete HS complex upon hydration and dehydration (Fig. 5, Dalton Trans. accepted.).
Development of calorimetric effect materials based on complex molecules
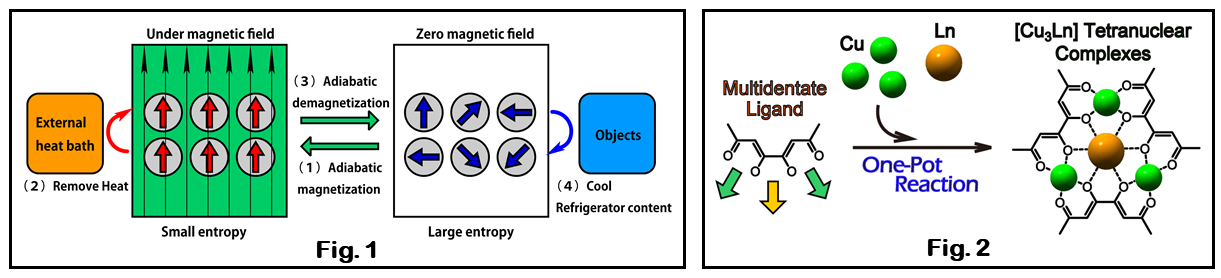
Cooling technology still uses the calorific effect based on gas compression, which has a high environmental load and is inefficient, and innovative technological developments are still required. Novel environmentally friendly and efficient refrigeration technologies using solid refrigerants are being investigated, with magnetic refrigeration effects in the low temperature range and cooling methods using balocalorimetric effects and electric calorimetric effects in the high temperature range attracting attention (Fig. 1).
We have been developing calorimetric effect materials based on highly designed coordination compounds, and have designed, synthesized, and evaluated molecular magnetocalorimetric materials for use in magnetic refrigeration. We are developing materials that exhibit a large magnetic entropy effect at the desired temperature and magnetic field, by appropriately arranging the spins of metal ions in close to each other. We have discovered that different kinds of metal ions can be integrated by using multidentate ligands, and have reported, for example, that [Cu3Ln] complexes such as those shown in Fig. 2 can be synthesized in a one-pot reaction (Dalton Trans. 2023.).